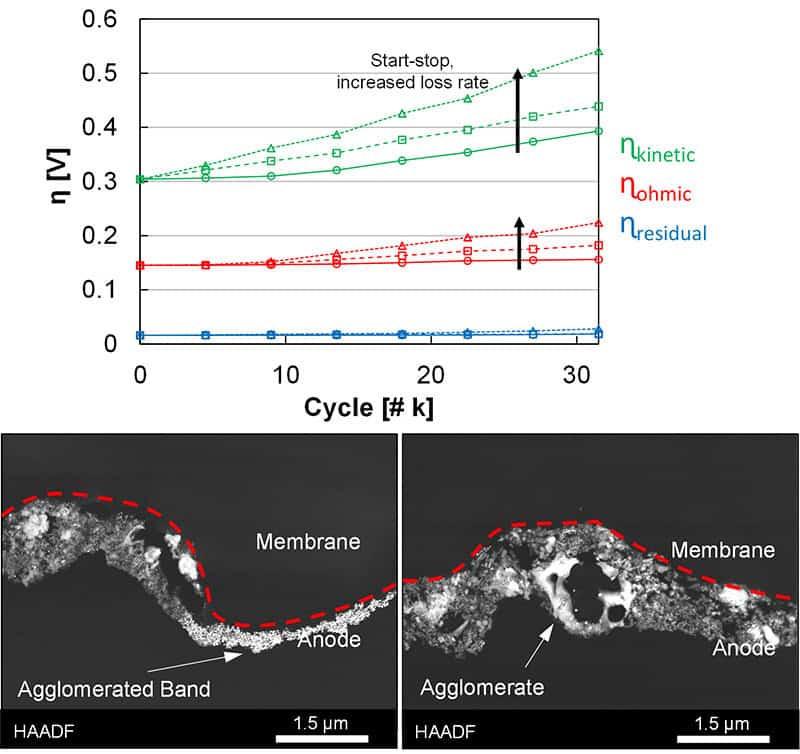
This webinar will detail recent efforts in proton exchange membrane-based low temperature electrolysis degradation, focused on losses due to simulated start-stop operation and anode catalyst layer redox transitions. Ex situ testing indicated that repeated redox cycling accelerates catalyst dissolution, due to near-surface reduction and the higher dissolution kinetics of metals when cycling to high potentials. Similar results occurred in situ, where a large decrease in cell kinetics was found, along with iridium migrating from the anode catalyst layer into the membrane. Additional processes were observed, however, and included changes in catalyst oxidation, the formation of thinner and denser catalyst layers, and platinum migration from the transport layer coating. Complicating factors, including the loss of water flow and temperature control were evaluated, where a higher rate of interfacial tearing and delamination were found. Current efforts are focused on bridging these studies into a more relevant field-test and include evaluating the possible differences in catalyst reduction through an electrochemical process versus hydrogen exposure, either direct or through crossover. These studies seek to identify degradation mechanisms and voltage loss acceleration, and to demonstrate the impact of operational stops on electrolyzer lifetime.
An interactive Q&A session follows the presentation.

Shaun Alia has worked in several areas related to electrochemical energy conversion and storage, including proton and anion exchange membrane-based electrolyzers and fuel cells, direct methanol fuel cells, capacitors, and batteries. His current research involves understanding electrochemical and degradation processes, component development, and materials integration and optimization. Within HydroGEN, a part of the U.S. Department of Energy’s Energy Materials network, Alia has been involved in low temperature electrolysis through NREL capabilities in materials development and ex and in situ characterization. He is further active within in situ durability, diagnostics, and accelerated stress test development for H2@Scale and H2NEW.
The post Start-stop operation and the degradation impact in electrolysis appeared first on Physics World.